Johnson & Johnson Fined $344 Million for Selling Transvaginal Mesh Implants Despite Health Warnings
Latest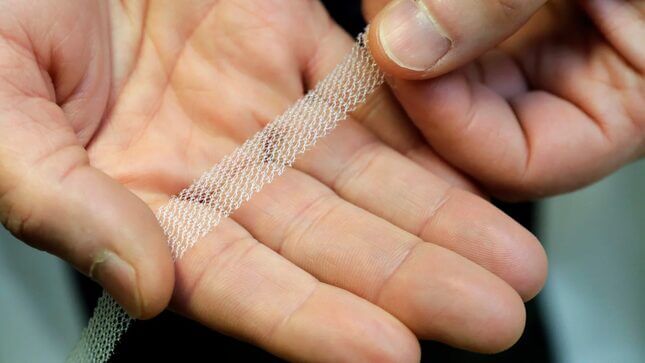

Johnson & Johnson has been fined $344 million for marketing and selling transvaginal mesh implants despite knowing the health risks, The Guardian reports. California state attorney general Xavier Becerra accused the company of knowing “the danger of its mesh products” and choosing to still put “profits ahead of the health of millions of women,” because, well, they were, and they did.
-

-

-

-

-

-

-

-

-

-

-

-

-

-

-

-

-

-

-

-

-

-

-

-

-

-

-

-

-

-

-

-

-

-

-

-

-

-

-

-








































