FDA Now Wants Textured Breast Implants to Be Recalled
Latest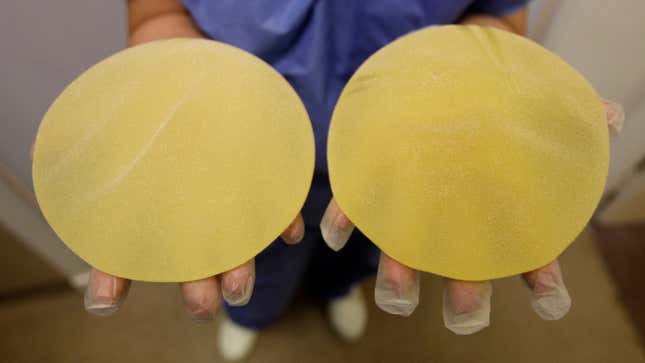

Almost three months after the Food and Drug Administration chose not to enact a wholesale ban of textured breast implants—a type of implant linked to a rare cancer—the agency has asked the breast implant maker Allergan to recall its textured implants currently available in the United States. According to the FDA, Allergan is “moving forward with a worldwide recall.”
Worldwide, textured breast implants have been linked to 573 cases of anaplastic large-cell lymphoma, and 33 people have died, according to updated numbers from the FDA. An overwhelming majority of both the cancer diagnoses—481—as well as the deaths in which the maker of the implant is known—12 out of 13—resulted from Allergan implants. “Based on the currently available information, including the newly submitted data, our analysis demonstrates that the risk of BIA-ALCL with Allergan BIOCELL textured implants is approximately six times the risk of BIA-ALCL with textured implants from other manufacturers marketing in the U.S,” the FDA wrote in its statement.
-

-

-

-

-

-

-

-

-

-

-

-

-

-

-

-

-

-

-

-

-

-

-

-

-

-

-

-

-

-

-

-

-

-

-

-

-

-

-

-








































